Understanding your industry challenges
In medical-surgical equipment factories, the constraints of comfort and air treatment are particularly stringent because of the strict quality and cleanliness standards required for the manufacture of medical equipment.
Temperature and humidity conditions must be maintained at specific levels to ensure the quality and stability of the materials used in medical devices.
Air handling systems incorporate very high efficiency filters to remove dust particles and contaminants from the air, to maintain cleanliness and air quality. Appropriate air circulation is therefore necessary to remove contaminants and ensure uniform temperature and humidity.
In addition, positive air pressure regulation can be implemented to prevent the entry of external contaminants.

This ETT innovation, combined with a heat pump, meets the need to reduce consumption while maintaining summer comfort.
The control system adapts the machine's operation in real time to optimise energy consumption.
The ULTI+ R32 OR range can be installed on a water loop. This system combines performance (SEER / SCOP, no defrosting, wide operating range) and accessibility (installation on standard upstand, installation in equipment room possible). Three hydraulic configurations are available.
Dehumidification in sales areas is essential for maintaining a comfortable and healthy indoor environment. By regulating humidity levels, it helps prevent the formation of condensation, which can damage products and materials, while ensuring the well-being of customers and staff.
Your project
New build
Refurbishment
In pictures
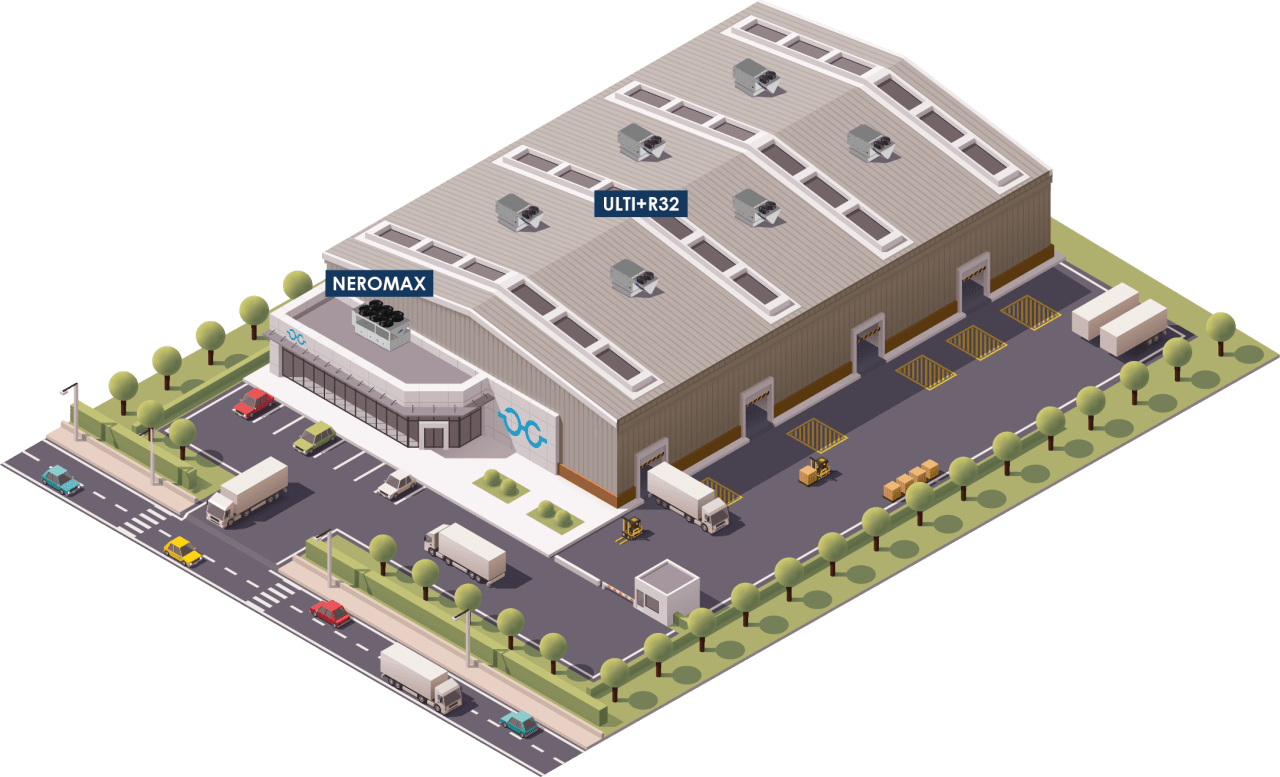
Dedicated solutions
Have a question ?
A project ?
High-performance, sustainable solutions for the medical-surgical sector
The medical devices market in France was estimated at around €14 billion.
The medical devices industry in France comprises more than 1,500 companies.
More than 85,000 people work in the medical devices sector in France.




Regulations
In France, the medical-surgical industry has to comply with a number of strict regulations on air treatment to guarantee the safety and quality of the products it manufactures. These regulations aim to maintain controlled environmental conditions to prevent contamination of medical devices.
Firstly, French standards generally require facilities to comply with strict air quality standards in production, storage and packaging areas. This includes rigorous control of airborne particles, humidity and airflow management to avoid cross-contamination. Plants often have to follow ISO 14644-1 guidelines for the classification of cleanrooms, defining levels of air cleanliness according to the type of production.
In addition, ventilation and filtration systems must be designed and maintained to ensure effective purification of the ambient air. Regular testing and audits are often required to verify ongoing compliance with standards.
Finally, the regulations also require traceability of production conditions, including control of the working environment to ensure the safety of employees and the quality of the medical products manufactured. These measures ensure that medical and surgical components meet international quality and safety standards.










